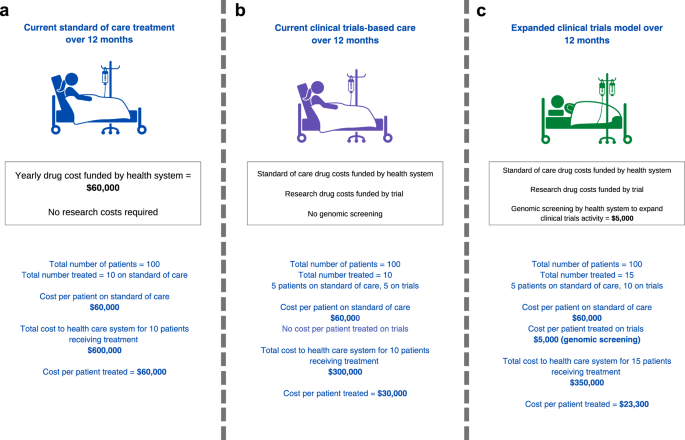
If the principle of patient benefit is accepted, does increased access to clinical trials make economic sense? The rising costs of new drugs has fundamentally changed the answer to this question. Let us assume that the health system bears standard-of-care treatment cost of $60,000 per patient per year, such that the total cost to the health system is $600,000 for 10 patients treated (Fig. 2a). If 5 of 10 patients access a drug provided for free by an industry partner through a trial, this provides a treatment cost-offset of $300,000 to the health system compared to standard-of-care drug access (Fig. 2b). Effectively, a fraction of the government pharmaceutical budget could be partially re-purposed to support the expansion of trials as a standard of care. Even if the health system was to invest an additional $5,000 per patient to support genomic screening and participation in clinical trials and cost per patient treated is $23,300, the system would still save $36,700 per patient assuming an additional 5 people would enrol and receive treatment through trials (Fig. 2c; compared to Fig. 2a, cost per patient treated is $60,000). The more people access therapies via clinical trials, the better for the economic benefit to the health system. To incentivize industry to conduct trials, the health system needs to contribute to making trials more efficient. For industry, an efficient increase in clinical trials participation reduces the length of time to conduct trials and associated costs.
For oncology drug development, health system-industry collaboration offers many opportunities for efficiencies and cost savings. Oncology drugs increasingly target biomarkers, often in the form of mutations detectable by genomic screening. Cancers are being increasingly subdivided therapeutically according to these biomarkers, generating the need to identify specific subpopulations carrying the cognate therapeutic biomarker for clinical trials. The cost of screening large numbers of patients to find these subgroups adds to the costs of drug development. A relevant drug target may be present in fewer than 1% of the general population, meaning that 100 people need to be screened to find one eligible participant. Currently, industry bears the costs of identifying such patients for clinical trials through diagnostic screening tests, almost invariably focusing on a single gene target, conducted separately for each trial and participating institution. Typically these single gene tests reflect the regulatory bodies’ requirement that a purpose-built companion diagnostic be approved with the therapy – a co-dependent technology.
The advent of comprehensive genomic profiling (CGP) can radically transform the efficiency of identifying subpopulations for clinical trials. CGP enables the screening of hundreds of potential drug targets in a single assay23,24, such that one test could be used to triage patients for dozens of trials. Realising the efficiencies of this approach requires the shift from trial-specific single gene testing, to CGP screening of patient populations on behalf of multiple trials.
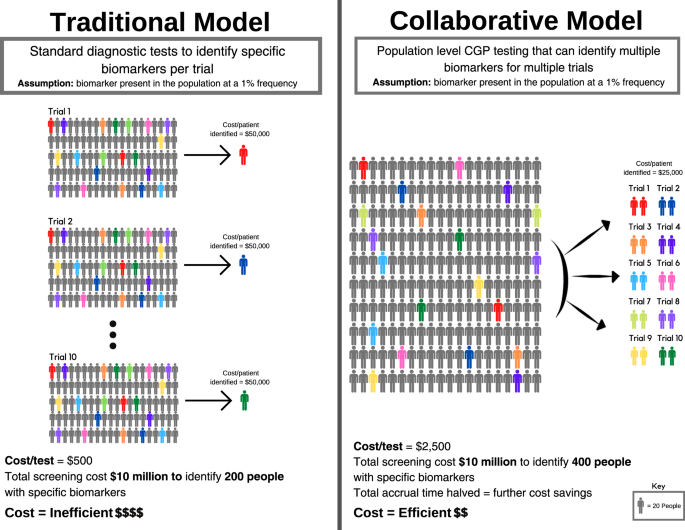
To illustrate this, consider the following hypothetical example of 10 companies intending to perform 10 trials (Fig. 3). Each trial is dependent on screening for a distinct biomarker present in the population at a 1% frequency. Each trial needs to screen 2,000 patients to identify 20 patients with the relevant biomarker (assuming for the purposes of argument 100% enrolment of suitable candidates onto each trial). Using a diagnostic screening test that costs $500 per individual, a 20-patient trial requires a $1 million screening budget (Fig. 3a). For a trial that costs $1 million to run (20 patients x $50,000 per patient enrolled), screening may account for half of the total cost. Collectively, 10 trials need to screen 20,000 patients using 10 biomarker-specific and purpose-built tests, with total screening expenditure of $10 million.
However, if the 10 biomarkers for these trials are mutually exclusive, then in principle only 2000 patients need to be screened to support all 10 trials through a single CGP test (Fig. 3b). If a CGP test costs $2500 per individual, screening 2000 individuals would cost $5 million to support 10 trials. Alternatively, using the original $10 million budget for all 10 trials, 4000 patients could be screened, identifying those patients twice as fast, thereby cutting the time to trial completion by half. In summary, the reduced costs of identifying eligible patients, and the acceleration of trials completion, are the twin drivers for decreasing the costs of drug development. Furthermore, CGP cost is declining, making population-level screening for guiding drug treatments more feasible.
Screening for 10 trials involving multiple industry partners, requires an ‘honest broker’ operating on behalf of all parties, with access to the patient populations to be screened. For these reasons, health systems, either directly or via an ‘honest broker’, are ideally placed to undertake biomarker screening in partnership with industry. The funding for CGP tests may be cost-efficient for health systems as patients might shift from system-reimbursed therapy to trial-based therapy.
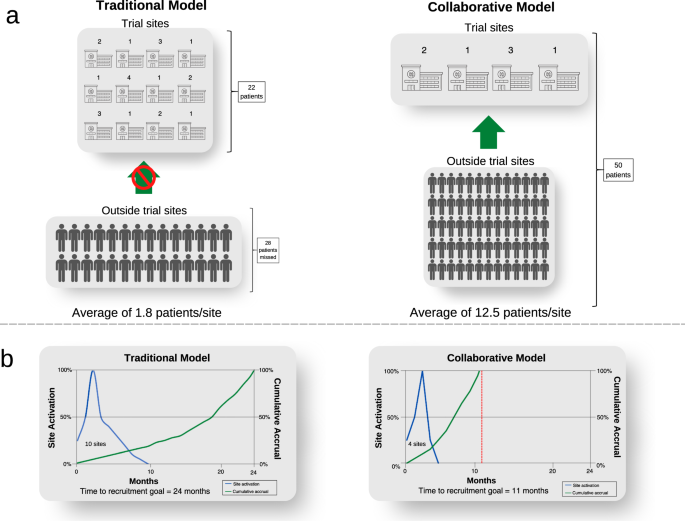
The collaborative model has an important additional benefit by expanding screening from sites where trials are conducted, to a much larger population across the entire health system (Fig. 4). Screening is limited to trial sites in the traditional model. The sponsor needs to open more trial sites to maximise the population to be screened because of the patient catchment area of the institution. If sites do not routinely undertake screening, this is funded by each trial. Opening each trial site adds costs and time related to governance and monitoring complexities. In contrast, the collaborative model would need fewer trial sites since the number of trial sites is predicated on the site trial capacity, not its patient catchment. To illustrate this point, for rare cancer populations, trials may not be feasible if the sponsor opens trial sites at 12 institutions to identify 22 patients for a trial, missing the opportunity of recruiting 28 people who are outside the trial sites (Fig. 4a). On the other hand, all 50 people could be identified by an independent population-based screening program in the collaborative model, who are referred to only four sites opened for trial conduct, increasing trial efficiency (Fig. 4b). This increases the clinical experience at each site, and the contribution of site investigators in answering the trial question. It also increases the system capacity for trials in total, by distributing the burden of trial conduct across a broader range of treating centres, whereas currently the burden of trials falls asymmetrically on high-volume centres, whose capacity may be saturated. Finally, it increases clinical trials engagement of centres which might otherwise not participate due to their patient volumes. Some of the cost savings outlined above could be reallocated to subsidies to support patient travel across a broader network of trial centres.
Importantly, the collaborative model will lead to better health outcomes for patients overall, for several reasons: (1) the total number of patients receiving biomarker-dependent therapies should increase compared with the existing model, due to enhanced access to trials; (2) the greater efficiency of CGP screening means that a greater fraction of patients carrying the relevant treatable biomarker will be identified than is currently the case; and (3) the greater speed and efficiency of trials conduct will reduce the net costs of drug development. In short, the greater the number of trials, the greater the amortization of the costs of CGP screening (or the greater the numbers of patients that can be screened with the same resource).
Source link